Search this article
Chemical vapor deposition research
Alicat has been cited in over 1,000 peer-reviewed research papers. The following papers focus on chemical vapor deposition and emerging technologies in that field. Contact us if you’d like your research to be highlighted.
Abstract
Extrapolating the properties of individual CNTs into macro-scale CNT materials using a continuous and cost-effective process offers enormous potential for a variety of applications. The floating catalyst chemical vapor deposition (FCCVD) method discussed in this paper bridges the gap between generating nano- and macro-scale CNT material and has already been adopted by industry for exploitation. A deep understanding of the phenomena occurring within the FCCVD reactor is thereby key to producing the desired CNT product and successfully scaling up the process further.
This paper correlates information on decomposition of reactants, axial catalyst nanoparticle dynamics, and the morphology of the resultant CNTs and shows how these are strongly related to the temperature and chemical availability within the reactor. For the first time, in-situ measurements of catalyst particle size distributions coupled with reactant decomposition profiles and a detailed axial SEM study of formed CNT materials reveal specific domains that have important implications for scale-up. A novel observation is the formation, disappearance and reformation of catalyst nanoparticles along the reactor axis, caused by their evaporation and re-condensation and mapping of different CNT morphologies as a result of this process.
Reference
Hoecker, C., Smail, F., Bajada, M., Pick, M., & Boies, A. (2015). Catalyst Nanoparticle Growth Dynamics and their influence on product morphology in a CVD process for continuous carbon nanotube synthesis. Carbon, 96, 116–124. https://doi.org/10.1016/j.carbon.2015.09.050
Abstract
The role of two sulfur sources, such as, thiophene and carbon disulfide, on the structure (graphitic layer) of carbon nanotube cotton (CNT-c) has been investigated during floating catalyst chemical vapour deposition (FC-CVD). The CNT-c assembly at 1200 °C with ferrocene and ethanol as catalyst and carbon precursors respectively was found to be predominantly consisting of (a) single-walled carbon nanotubes (SWCNTs) in the presence of carbon disulfide and (b) multi-walled carbon nanotubes (MWCNTs) in the presence of thiophene. The finding was based on detailed characterization using Raman spectroscopy, transmission electron microscopy and scanning electron microscopy. A possible mechanism for having two different structures has been discussed. The experimental results demonstrated that the interaction of sulfur source with the catalyst particle strongly influenced the number of graphitic layers of CNTs in the CNT-c assembly.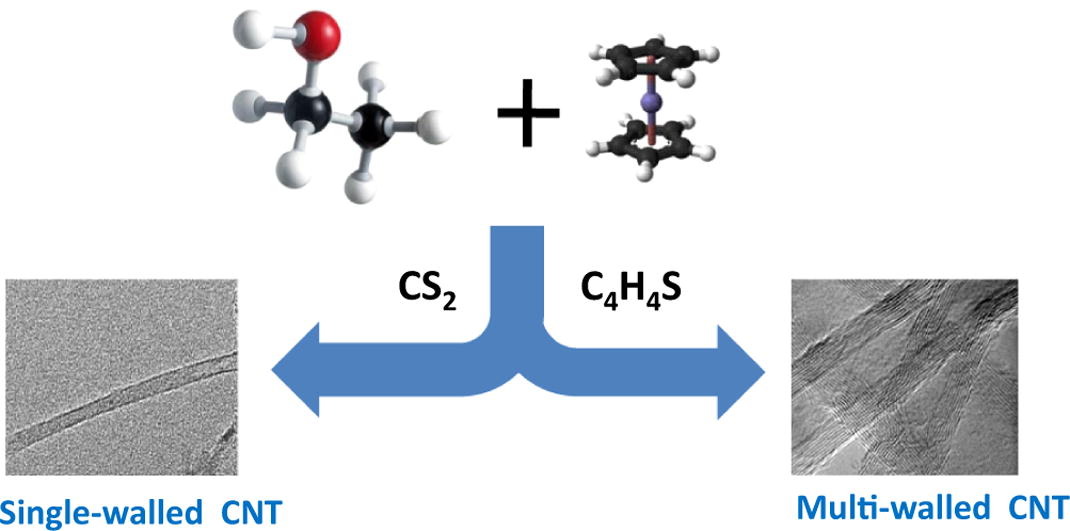
Reference
Yadav, M. D., & Dasgupta, K. (2020). Role of sulfur source on the structure of carbon nanotube cotton synthesized by floating catalyst chemical vapour deposition. Chemical Physics Letters, 748. https://doi.org/10.1016/j.cplett.2020.137391
Abstract
The floating catalyst chemical vapor deposition (FC-CVD) method is unique in providing the capability for continuous carbon nanotube (CNT) synthesis at an industrial scale from a one-step continuous gas-phase process. Controlling the formation of the iron-based catalyst nanoparticles is widely recognized as a primary parameter in optimizing both CNT product properties and production rate. Herein the combined influences of pyrolytic carbon species and catalytic nanoparticles are both shown to influence CNT aerogel formation. This work studies the source of carbon in the formed CNTs, the location of aerogel formation, the in-situ behavior of catalyst nanoparticles and the correlated morphology of the resultant CNTs.
Axial measurements using isotopically-labelled methane (CH4) demonstrate that carbon within all CNTs is primarily derived from CH4 rather than some of the early-forming CNTs being predominantly supplied with carbon via thermal decomposition of catalytic precursor components. Quantification of CNT production along the axis of the reactor definitively dispels the notion that injection parameters influence CNT formation and instead shows that bulk CNT formation occurs near the reactor exit regardless of the carbon source (CH4, toluene or ethanol). Supply of carbon to different reactor locations indicates that CNT aerogel formation will occur even when carbon is delivered near the exit of the reactor so long as the carbon source reaches a sufficient temperature (>1000 °C) to induce pyrolysis. These results give an indication of how future large-scale CNT reactors may be optimized and controlled by modifying downstream catalyst and carbon delivery.
Reference
Hoecker, C., Smail, F., Pick, M., & Boies, A. (2017). The influence of carbon source and catalyst nanoparticles on CVD synthesis of CNT aerogel. Chemical Engineering Journal, 314, 388–395. https://doi.org/10.1016/j.cej.2016.11.157
Abstract
Laser micro-Raman spectroscopy is an ideal tool for assessment and characterization of various types of carbon-based materials. Due to its special optical properties (CrN) coated stainless steel substrates. NCD films have been investigated by laser micro-Raman spectroscopy. The fingerprint of diamond based materials is in the spectral region of 1000-1600 cm-1 in the first order of Raman scattering spectrum.
By using of Gaussian peak fitting, characteristic peaks in the micro-Raman spectrum of NCD films including diamond peak (D), NCD features, a vibrational density of states (VDOS) in the ultra-nanocrystalline diamond (UNCD) clusters, graphitic (G) band and disordered (D) band can be assigned. These peaks and bands can be broadened, shifted in the spectral region or may be eliminated from the spectra due to NCD films grain sizes, synthesis conditions and other surface effects on the crystals. The increasing grain sizes to about 100 nm and faceted grains as the most important parameters can promote the diamond Raman signal, eliminate the VDOS, UNCD and even NCD features in the Raman spectrum.
Reference
Motahari, H., & Malekfar, R. (2019). Laser micro-raman spectroscopy of CVD Nanocrystalline Diamond Thin film. International Journal of Optics and Photonics, 13(1), 3–12. https://doi.org/10.29252/ijop.13.1.3