Search this article
Battery development research
Alicat has been cited in over 1,000 peer-reviewed research papers. The following papers focus on battery development and emerging technologies in that field. Contact us if you’d like your research to be highlighted.
Abstract
We report a high-yield single-step method for synthesizing nitrogen-doped graphene nanostripes (N-GNSPs) with an unprecedentedly high percentage of pyridinic-type doping (>86% of the nitrogen sites), and investigate the performance of the resulting N-GNSPs as a lithium-ion battery (LIB) anode material. The as-grown N-GNSPs are compared with undoped GNSPs using scanning electron microscopy (SEM), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), helium ion-beam microscopy (HIM), and electrochemical methods.
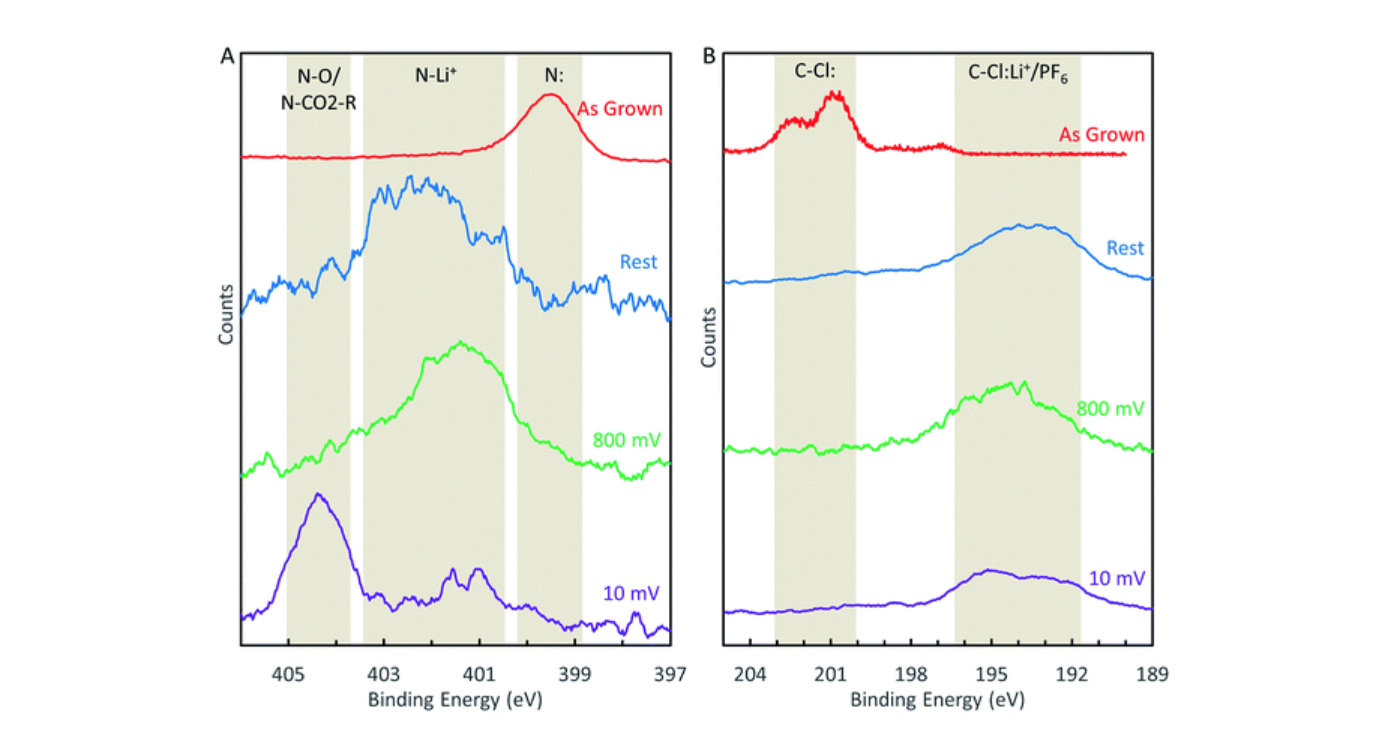
(A) Evolution of the N-1s XPS peak during LIB cycling. (B) Evolution of the Cl-2p XPS peak during LIB cycling
As an anode material we find that pyridinic-type N-GNSPs perform similarly to undoped GNSPs, suggesting that pyridinic sites alone are not responsible for the enhanced performance of nitrogen-doped graphene observed in previous studies, which contradicts common conjectures. In addition, post-mortem XPS measurements of nitrogen-doped graphene cycled as a lithium-ion battery anode are conducted for the first time, which reveal direct evidence for irreversible chemical changes at the nitrogen sites during cycling. These findings therefore provide new insights into the mechanistic models of doped graphene as LIB anodes, which are important in improving the anode designs for better LIB performance.
Reference
Bagley, J. D., Kumar, D. K., See, K. A., & Yeh, N.-C. (2020). Selective formation of pyridinic-type nitrogen-doped graphene and its application in lithium-ion battery anodes. RSC Advances, 10(65), 39562–39571. https://doi.org/10.1039/d0ra06199a
Abstract
The current paper presents an innovative route for selective lithium extraction, followed by production of battery grade LiOH·H2O via reductive hydrogen roasting, water leaching and LiOH·H2O crystallization. The results suggest that during the initial hydrogen reduction stage, almost 98% of Li can be transformed into soluble LiOH·H2O with H2 reduction at 500 °C within 15 min, while Ni, Co, Mn all transform into their corresponding insoluble metals or their oxides.
Consequently, almost all of Li present in the roasted material can be effectively separated from other impurities by 10 min of water leaching at 25 °C with liquid-solid (L/S) ratio of 2, such that the extraction of other metals like Ni, Co, Mn are < 0.1%. Subsequent stages allow high purity LiOH·H2O (99.92%) to be obtained directly through evaporation and crystallization. In addition, high nickel battery cathode materials (LiNi0.8Co0.1Mn0.1O2) are prepared from the recycled LiOH·H2O products and these demonstrate good electrochemical performance. Overall, this newly developed hydrogen reduction-based process may provide a more simple, efficient, and environmentally friendlier method for the recovery of valuable metals from spent LIBs, as well as offering great potential for straightforward industrial-scale recycling.
Reference
Liu, F., Peng, C., Ma, Q., Wang, J., Zhou, S., Chen, Z., Wilson, B. P., & Lundström, M. (2021). Selective lithium recovery and integrated preparation of high-purity lithium hydroxide products from spent lithium-ion batteries. Separation and Purification Technology, 259, 118181. https://doi.org/10.1016/j.seppur.2020.118181