- Blog
- Introduction to hydrogen fuel cells
Introduction to hydrogen fuel cells
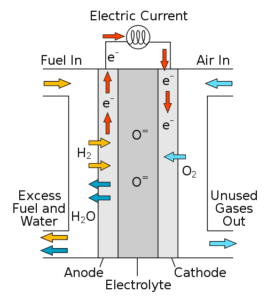
Hydrogen fuel cell example
Hydrogen fuel cells are a clean, emissions-free alternative to traditional combustion processes, and use hydrogen to generate energy via electrochemical reactions. These cells are made even more environmentally friendly when the hydrogen is produced via sustainable means.
There are many variations in fuel cell technology, but they share similar features. All fuel cells house an anode and cathode on either side of an electrolyte. In a proton-conducting fuel cell, hydrogen is fed to the anode and a catalyst is used to generate positively charged ions that flow through the electrolyte to the cathode. This induces a current, thereby generating electricity. Meanwhile at the cathode, air is fed into the system and combines with a catalyst, hydrogen ions, and electrons to produce heat and water as byproducts.
Types of fuel cells
The fundamental principle of the fuel cell was first proposed by Sir William Grove in 1838, and many deviations of the fuel cell have developed since then. Here we highlight some of the most prominent fuel cells and their respective applications.
Alkaline fuel cells (AFCs)

Apollo mission lunar module
Alkaline fuel cells are the oldest commercialized fuel cell, first invented by Francis Bacon in 1932. AFCs contain porous electrodes saturated with alkaline solution that are separated by a hydroxide electrolyte. Since they are highly sensitive to CO2, their reactions use pure oxygen or highly purified air. These are famous for their use by NASA in the 1960s to generate electricity and water for the crew of the Apollo and Space Shuttle programs. Because they are so well developed, alkaline fuel cells are currently the cheapest variant to manufacture.
Solid oxide fuel cells (SOFCs)
Solid oxide fuel cells contain a solid ceramic electrolyte layer that is heated to very high temperatures (1000°C, 1800°F) and conducts oxygen ions. Their primary use is in stationary power generation systems for small residential and business applications. While SOFCs are compatible with many fuel types and offer long-term stability, the high operating temperature creates challenges in terms of material compatibility and lengthy start-up times.
Proton exchange membrane fuel cells (PEMFCs)

PEM fuel cell stack testing
Proton exchange membrane fuel cells use a special electrolyte membrane that conducts protons between the anode and cathode. PEMFCs have higher power density, lower weight, and smaller volume than other fuel cells, making them ideal for vehicle and transport applications in addition to stationary applications. These are a fairly new development, and research efforts are focused on increasing viability by decreasing cost and increasing efficiency.
Molten carbonate fuel cells (MCFCs)
Molten carbonate fuel cells operate at high-temperatures like SOFCs, although they use a molten carbonate salt mixture electrolyte. MCFCs are being developed for electrical utility, military, and industrial applications.
Phosphoric acid fuel cells (PAFCs)
Phosphoric acid fuel cells have utilized phosphoric acid as the electrolyte since their introduction in 1961. These cells are primarily used in stationary power generation applications, particularly those within an output power range of 100-400 kW. An advantage is their ability to handle high levels of impurities compared to PEMFCs.
More on fuel cell electric vehicles
Fuel cell electric vehicles (FCEVs) are a major area of developmental focus, discussed frequently in popular media. These vehicles operate on fuel cell generated energy rather than standard combustion engine technology. FCEVs like the Toyota Mirai have competitive driving ranges compared to electric vehicles, and are very quick to refill. However, some challenges must be overcome before FCEVs are able to scale effectively – high price tags and insufficient hydrogen infrastructure.
Fun Fact! October 8th is National Hydrogen and Fuel Cell Day in the United States. The date was chosen based on the atomic weight of hydrogen: 1.008 amu.